|
Sample
Test Questions Grades 9 and 10
|

1. The table shows the solubility of lead nitrate in water. The units are grams of lead nitrate in 100 grams of water.
| Temperature °C | 20 | 40 | 60 | 80 | 100 |
| Solubility g/100g water | 58 | 74 | 94 | 115 | 140 |
Plot a graph of the solubility of lead nitrate in water using the axes provided.
|
|
Use it to answer the questions which follow.
(i) What happens to the solubility of lead nitrate as the temperature increases? ___________________________________________________________
(ii) What is the solubility of lead nitrate, in 100g of water, at
30 °C _______________________________________________
50 °C _______________________________________________
(iii) A saturated solution of lead nitrate was made at 50 °C using 100 g of water, and this was then allowed to cool to 30 °C. What happened as the solution cooled? ___________________________________________________________ ___________________________________________________________
ANSWERS: (i) It becomes more soluble.(ii) ~61g and ~84g (iii) The lead nitrate solidifies.
2. Everyday experiences can often be explained using scientific ideas about the way particles are arranged in solids, liquids and gases. (a) In the boxes below, show how the particles are arranged in a typical solid, liquid and gas (in each case, use the symbol X to represent each particle).
|
solid |
liquid |
gas |
(b) When a jar of coffee is opened, people in all parts of the room soon notice the smell. Use ideas about particles to explain how this happens. _________________________________________________________________ _________________________________________________________________
(c) Salt soon disappears when stirred in water. Explain why all parts of the water then taste salty. ____________________________________________________________ ________________________________________________________________
ANSWERS: (b) (c) Both answers should include something about diffusion of particles.
3. An element may be shown as
| 27 | Al |
| 13 |
How many electrons does one atom of this element contain?
A 13
B 14
C 27
D 40
ANSWER: A
4. What process changes a chlorine atom, Cl, into a chloride ion, Cl-?
A electron gain
B electron loss
C proton gain
D proton loss
ANSWER: A
5. Which equation represents the combustion of hydrogen gas?
A H2 + O2 = H2O2
B H2 + O2 = H2O
C 2H + O = H2O
D 2H2 + O2 = 2H2O
ANSWER: D
6. The equation for the burning of butane is shown below.
2C4H10 + 13O2 ÷ 8CO2 + 10H2O
How many moles of water are formed when one mole of butane burns completely?
A 4
B 5
C 8
D 10
ANSWER: B
7. An element has the electronic structure 2.8.3. It may be deduced that the element
A is in group 2 of the Periodic Table
B is in group 8 of the Periodic Table
C is in group 3 of the Periodic Table
D has a relative atomic mass of 11
ANSWER: C
8. What is added to water to prevent tooth decay?
A bacteria
B dissolved salts
C fluorides
D chlorine
ANSWER: C
9. acid + alkali = salt + .......................................
What is missing from the above equation?
A carbon dioxide
B hydrogen
C oxygen
D water
ANSWER: D
10. How should the following reaction be written as a balanced symbol equation?
carbon + carbon dioxide = carbon monoxide
A C + CO2 = 2CO
B C + CO2 = C2O2
C 2C + CO2 = 2CO
D 2C + CO = 2CO2
ANSWER: A
11. Gases, unlike solids, may be easily compressed because
A gas molecules are softer than those in solids
B gas molecules are smaller than those in solids
C gas molecules can move but those in solids cannot
D gas molecules are far apart but those in solids are touching
ANSWER: D
12. Chlorine is added to the domestic water supply in order to
A soften the water
B kill any germs
C improve the taste
ANSWER: B
13. Which of the following is a noble gas?
A argon
B carbon dioxide
C hydrogen
D oxygen
ANSWER: A
14. The table shows the densities of some Group 1 metals.
| metal | density in g/cm3 |
| A lithium | 0.53 |
| B sodium | 0.97 |
| C potassium | 0.86 |
| D rubidium | 1.53 |
Which metal sinks in benzene (density of liquid = 0.88 g/cm3) but floats in nitrobenzene (density of liquid = 1.2 g/cm3)?
ANSWER: B
15. The table shows the electronic structures of hydrogen and helium.
|
element
|
proton (atomic) number
|
number of electrons in:
|
||
| shell 1 | shell 2 | shell 3 | ||
| hydrogen | 1 | 1 | 0 | 0 |
| helium | 2 | 2 | 0 | 0 |
| lithium | 3 | ? | ? | ? |
What is the electronic structure of lithium?
shell 1 shell 2 shell 3
A 1 1 1
B 1 2 0
C 2 0 1
D 2 1 0
ANSWER: D
16. What happens when ice melts?
A Water molecules change to hydrogen and oxygen atoms.
B Water molecules change to water atoms.
C Irregularly arranged molecules change to regularly arranged molecules.
D Regularly arranged molecules change to irregularly arranged molecules.
ANSWER: D
17. This table shows the melting points and boiling points of four substances.
| substance | melting point /°C | boiling point / °C |
| A | - 203 | - 17 |
| B | - 25 | - 50 |
| C | 11 | 181 |
| D | 463 | 972 |
a.) Which substance is liquid at 100 °C? ___________
b.) Hydrogen reacts explosively with oxygen to form water. Use a diagram to show the bonding in a water molecule. Draw the outer electrons only (ie. valency electrons).
ANSWER: a.) C b.)
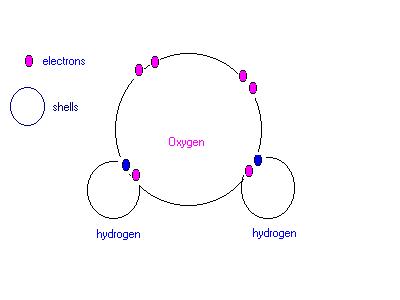 |
18. What causes the intake or release of energy in an exothermic or endothermic reaction?
A Electrons escaping or being drawn in
B Protons colliding during the reaction
C Bond making and breaking
ANSWER: C
19. What is the equilibrium of a reversible reaction?
A The point at which the yield in each direction is the same
B The point at which the yield of each reactant is the same
C The point at which the rate of reaction in each direction is the same
ANSWER: A
20. A pure substance which cannot be split up by chemical reaction is called...
A a compound
B a mixture
C an element
ANSWER: C
21. Elements that have been mixed can still be separated
A True
B False
ANSWER: A
22. Which of the following is NOT a property of a compound?
A It is difficult to separate out the elements in it
B Energy is often released or taken when it is formed
C Proportions of the different elements can vary
ANSWER: C
23. Which of these statements about the periodic table is untrue?
A Elements are arranged in increasing order of atomic number
B Elements with similar properties are arranged in columns
C The heaviest elements are at the top-left of the table
D It was developed in part by Mendeleev
ANSWER: C
24. What are the horizontal rows of elements in the periodic table called?
A Groups
B Periods
C Transition elements
D Halogens
ANSWER: B
25. How many electrons do group VI elements have in their outer shell?
A It varies
B 4
C 6
D none
ANSWER: C
26. When group I elements react, they will...
A gain two electrons
B gain one electron
C not gain or lose any electrons
D lose one electron
E lose two electrons
ANSWER: D
27. What is the name given to water loss to the atmosphere by green plants?
A Evaporation
B Transpiration
C Condensation
D Distillation
ANSWER: B
28. Which of these statements about water uses is FALSE?
A Water is used as a coolant
B Water is used as a raw material to produce hydrogen
C Water is used as a raw material to produce carbon
D Water is used as a solvent
ANSWER: C
29. What are gases that contribute to global warming called?
A warming gases
B natural gases
C greenhouse gases
D fossil fuels
ANSWER: C
30. Which of the sections of the Earth`s core is molten?
A Outer
B Inner
C Both
ANSWER: B
31. Tectonic plates sliding past one another can cause...
A volcanoes
B constructive plate margins
C destructive plate margins
D earthquakes
ANSWER: D
32. In single-celled organisms, gaseous exchange takes place through
the cell membrane
A True
B False
ANSWER: A
33. How are lungs adapted for efficient gaseous exchange?
A They have thin, moist membranes with a large surface area
B They have thick membranes with large cells and a shallow diffusion gradient
ANSWER: A
34. How do mammals respire?
A Aerobically
B Anaerobically
C Both aerobically and anaerobically
ANSWER: A
35. Enzymes are...
A Proteins
B Chloroplasts
C Genes
D Mitochondria
ANSWER: A
36. Can enzymes help to speed up reactions?
A Yes
B No
ANSWER: A
37. Which of these factors does NOT speed up transpiration?
A High temperature
B Sunlight
C Low humidity
D Wind
E High pressure
ANSWER: B
38. What do phloem vessels do?
A Transport food through a plant
B Transport water through a plant
C Hold chlorophyll
D Transport energy
ANSWER: A
39. In addition to transporting water, what other function does a xylem vessel perform?
A Gives strength to the stem
B Stores energy
C Stored food
D Transports food
ANSWER: A
40. Does an organism which reproduces asexually need another organism to reproduce with?
A Yes
B No
ANSWER: B
41. Is the offspring of an asexual organism genetically identical to its parent?
A Yes
B No
ANSWER: A
42. Cross-pollination takes place solely within one individual plant
A True
B False
ANSWER: B
43. What are the two main functions of fruit for a plant?
A To disperse and protect the seeds
B To attract insects and to protect the seeds
C To disperse the seeds and attract insects
D To store food for itself and to protect seeds
ANSWER: A
44. Put these in order of size, smallest first:
A Gene, cell, chromosome, base, nucleus
B Base, gene, chromosome, nucleus, cell
C Gene, chromosome, base, nucleus, cell
D Chromosome, base, gene, nucleus, cell
E Base, chromosome, nucleus, gene, cell
ANSWER: B
45. Which of these cannot be a result of genetic mutation?
A Cancer
B An increased chance of survival
C Twins
D Sickle-cell anaemia
ANSWER: C
46. If the climate changes very suddenly, what is the most likely effect on species which live in and are adapted to the previous climate?
A Immediate evolution of the species to adapt to the conditions
B Extinction of the species
C No effect
ANSWER: B
47. Evolution can lead to the creation of new species
A True
B False
ANSWER: A
48. Evolution takes place...
A In the space of one generation
B Over a large number of generations
C Only over millions of years
ANSWER: B
49. Which of the following did Darwin specify to be cruicial to the Theory of Evolution?
A Survival of the fittest of each species
B Extinction due to new predators or disease
C Death and decay of animals
ANSWER: A
50. What effect does temperature have on enzymes?
A Boiling will denature them, as will being too cold
B Boiling will not harm them, but being too cold will denature them
C Boiling and cooling will both reduce the speed of their action
D Boiling will denature them, but cooling will only slow down their work
ANSWER: A
51. The laws of reflection at plane surfaces include...
A the angle of incidence equals the amplitude
B the angle of incidence equals the angle of reflection
C the angle of reflection equals the convergence
ANSWER: B
52. The image produced by a pinhole camera is always...
A Black and white
B Fuzzy
C Inverted
D Bright
ANSWER: C
53. An object on Earth has a weight of 420 N and a mass of 42 kg. What is the mass and weight of the object on the moon, if the moon's gravitational attraction is one sixth that of earth?
| Weight (N) | Mass (kg) | |
| A | 42 | 420 |
| B | 70 | 42 |
| C | 84 | 42 |
| D | 42 | 70 |
ANSWER: B Comment: Mass remains constant and does not change. Weight however changes according to the gravitational field strength. Therefore weight = 420 × 1/6 = 70 N
54. When an object is placed in a liquid (water) it displaces a volume of liquid equal to ________________.
ANSWER: its own volume. Comment: If an object is placed in a liquid, the level of the liquid rises by the same volume as that of the object. This is a useful way of determining the volume of objects with irregular shapes.
55. An athlete runs 240 m in 30 seconds. His average speed (in m.s-1) is
A 0.125
B 8
C 80
D 7200
ANSWER: B Comment: Average speed = distance/time = 240m/30s = 8 m.s-1
56. The pressure in N.m-2 exerted by a 60 kg person standing on a floor where the contact area is 100 cm2 is:
A 0.6 kPa
B 6 Pa
C 60 Pa
D 60 kPa
ANSWER: C Comment: P = F/A = 600 N/0.01 m2 = 60 000 Pa = 60 kPa Note: force = weight exerted by a person = mass × 10 = 60 kg × 10 = 600 N (Area of 100 cm2 = 0.01 m2)
57. A stone dropped from a helicopter takes 10 seconds to hit the sea below. What is its final velocity? (Assume that the acceleration due to gravity is 10 m.s-2)
A 10 m/s
B 100 m/s
C 0 m/s
D 1 m/s
ANSWER: B Comment: velocity = acceleration × time = 10 m.s-2 × 10 s = 100 m.s-1 (The acceleration of the falling stone is taken as the acceleration due to gravity i.e. 10 m.s-2.)
58. Which one of the following is a non-renewable source of energy?
A Wind
B Uranium
C The sun
D Tides
ANSWER: B Comment: There are limited deposits of uranium on the earth.
59. A radio converts:
A electrical energy into light energy.
B chemical energy to electrical energy.
C electrical energy into heat.
D electrical energy into sound energy.
E potential energy to kinetic energy.
ANSWER: D Comment: Most energy changes involve energy changing from one form into two or more different forms. Electrical energy is mainly converted into sound via sound waves.
60. Which of the following are advantages of hydro-electric power stations over coal power stations?
A Use of renewable energy sources and pollution free
B Cheaper running costs with pollution effects
C More expensive to operate with no pollution effects
D Use of non-renewable energy sources and pollution free
ANSWER: A Comment: The use of renewable energy sources and the fact that there is no sulphur or carbon dioxide being released into the atmosphere. The running costs are also cheaper.
61. What is the reading on ammeter C? (Assume the bulbs are identical)
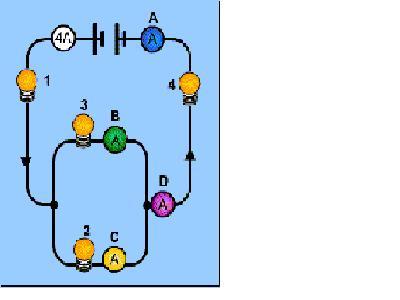
A 1 A
B 2 A
C 3 A
D 4 A
ANSWER: B Comment: The current splits in half because the lamps offer equal resistance.
62. The colour of a pure red rose in white sunlight is a result of the fact that:
A the rose absorbs red light only.
B the rose absorbs all white light.
C the rose reflects only red light.
D the rose reflects all light except red.
ANSWER: C Comment: Red light is the only component of the visible spectrum which reaches the eye.
63. A ray of light strikes the surface of a mirror at an angle of incidence of 40°. The angle of reflection is:
A 40°
B 90°
C 50°
D 0°
ANSWER: A Comment: The law of reflection: the angle of incidence is equal to the angle of refraction.
64. White light passes through a triangular prism and a band of colour is observed where it emerges. The phenomenon that occurs in the prism and the band of colour are respectively known as:
A dispersion and spectrum.
B spectrum and refraction.
C spectrum and dispersion.
D dispersion and refraction.
ANSWER: A Comment: The splitting up of white light into its component colours is known as dispersion and the band of colour is called the visible spectrum.
65. Which one of the following is not a property of images formed in a plane mirror?
A They are laterally inverted
B They are enlarged
C They are as far behind the mirror as the object is in front of it
D They are virtual
ANSWER: B Comment: The image in a plane mirror is the same size as the object.
